Narragansett Bay Adult Oyster DNA Extractions - Part 4
DNA Extraction of Adult Oyster Tissue - Part 4
~10 wild adult oysters were collected from 5 populations in Narragansett Bay
- Narrow River
- Green Hill Pond
- Barrington River
- Kickemuit
- Mary C. Donovan Marsh
Mantle and gill tissue were dissected from the oysters. Dissection protocol can be accessed here.
DNA Extractions
Completed on January 31, 2021
Zymo Research Quick-DNA Miniprep Plus used for DNA extractions of adult oyster tissue
- Pull samples out of -80 freezer and put on ice
- NAR_3 M1
- BAR_6 M1
- BAR_10 M1
- KIC_9 M1
- MCD_7 M1
- Pull Proteinase k out of upright -20 freezer in Puritz Zymo Reagents box and put on ice
- Pull out Blue Solid Tissue Buffer and Nuclease-free water
- In 2 ml Homogenization Tubes with ceramic beads, add 190 ul of Blue Solid Tissue Buffer and 190 ul of Nuclease-free water
- Vortex and spin down
- Sterilize forceps with 10% Bleach, Type II DI Water, and 70% EtOH
- Use forceps to transfer tissue piece from original tube to 2 ml Homogenization tube with ceramic beads at solid tissue buffer and nuclease-free water
- Sterilize forceps before each sample
- Setup tissuelyser II
- Make sure to balance both tube racks
- Homogenize tissue samples in tissuelyser II for 2 minutes at 30 Hz
- This will create a ton of bubbles in the sample tubes
- Let the samples sit for about 3 minutes then spin down on mini centrifuge to bring down bubbles
- Add 20 ul of Proteinase k to each sample
- Vortex for 10 seconds then spin down on mini Centrifuge
- Put samples in thermomixer at 55 deg C at 600 rpm for 30 minutes
- Halfway thru, spin down samples on mini centrifuge
- Added 15 more minutes at 1000 rpm because samples were cloudy/mucousy
- Make new set of labeled 1.5 ml tubes
- After 30 minute incubation, place all samples in tabletop centrifuge and spin at 13000 rcf for 2 minutes
- Transfer all supernatant (400 ul) to the newly labeled 1.5 ml tubes
- Make 1.5 ml tube of 10 mM Tris HCl pH 8 and place in thermomixer at 70 deg C
- Set up tubes for extraction - 1 yellow spin column inside a collection tube for each sample - label lid of spin column
- Get liquid waste beaker from sink near -80 freezers
- Add 2 parts volume (800 ul) of Genomic Binding buffer to each tube
- Vortex for 5 seconds and spin down in mini centrifuge
- Add 800 ul of sample to their labeled yellow spin column
- Centrifuge spin columns at 13000 rcf for 1 minute
- Pour off flow through in liquid waste beaker
- Put spin columns in same collection tubes
- Add remaining liquid (400 ul) from each sample to labeled yellow spin column
- Centrifuge spin columns at 13000 rcf for 1 minute
- Pour off flow through in liquid waste beaker
- Transfer spin columns to new collection tubes and discard of old collection tubes
- Add 400 ul of DNA pre-wash buffer to each spin column
- Centrifuge at 13000 rcf from 1 minute
- Pour off flow through in liquid waste beaker
- Place spin columns in same collection tubes
- Add 700 ul of g-DNA wash buffer to each spin column
- Centrifuge spin columns at 13000 rcf for 1 minute
- Pour off flow through in liquid waste beaker
- Put spin columns in same collection tubes
- Add 200 ul of g-DNA wash buffer to each spin column
- Centrifuge spin columns at 13000 rcf for 1 minute
- Make final 1.5 ml tubes - label lid with sample id, elution #, and DNA; label side with initials, date of extraction, sample id, elution #, DNA, and C. virginica
- Make 2 tubes for each sample - splitting the two elutions into two separate tubes
- Transfer spin columns to first set of labeled 1.5 ml tubes
- Pour off flow through in liquid waste beaker and discard collection tubes
- Take warmed 10 mM Tris HCl pH 8 out of thermomixer
- Add 10 ul of warmed 10 mM Tris HCl pH 8 to each spin column by dripping directly over the filter without touching it
- Immediately place tubes in centrifuge with all the lids of the 1.5 ml tubes facing the same direction and centrifuge at MAX speed for 1 minute
- No incubation period for the first elution
- Take tubes out - DO NOT pour off liquid, keep in tube
- Transfer spin columns to second set of labeled 1.5-ml tubes, add 100 ul of warmed 10 mM Tris HCl pH 8 to each spin column by dripping directly over filter without touching it
- Put first set of 1.5-ml tubes with the first elution on ice
- Incubate for 15 minutes
- Centrifuge at max speed for 1 minute
- Take out spin columns at discard
- Make labeled PCR strip tubes - 1 for each elution = 2 per sample
- Add 2 ul of DNA to the respective PCR tube for agarose gel
- 1 ul will be taken directly from 1.5-ml tube for Qubit
7 ul remaining in all E1 1.5 ml tubes; 97 ul remaining in all E2 1.5 ml tubes
Qubit dsDNA BR assay
The captured pools were quantified following Qubit protocol for BR DNA
| Sample | Avg ng/μl |
|---|---|
| Std 1 | 183 RFU |
| Std 2 | 22061 RFU |
| NAR_3 E1 | 290 |
| NAR_3 E2 | 23.0 |
| BAR_6 E1 | 129 |
| BAR_6 E2 | 9.05 |
| BAR_10 E1 | 78.0 |
| BAR_10 E2 | 9.34 |
| KIC_9 E1 | 146 |
| KIC_9 E2 | 13.9 |
| MCD_7 E1 | 77.5 |
| MCD_7 E2 | 9.30 |
This time around, there’s a lot more DNA in both elutions, with the first elution having a ton of smaller fragments of DNA.
Agarose Gel Electrophoresis
The DNA quality and size were assessed following Agarose Gel Protocol for a small 1% gel.
- Only 2 ul of DNA for each sample were used
- Did not use diluted gelgreen this time
- Used a different loading dye - Purple loading dye versus Tritrack
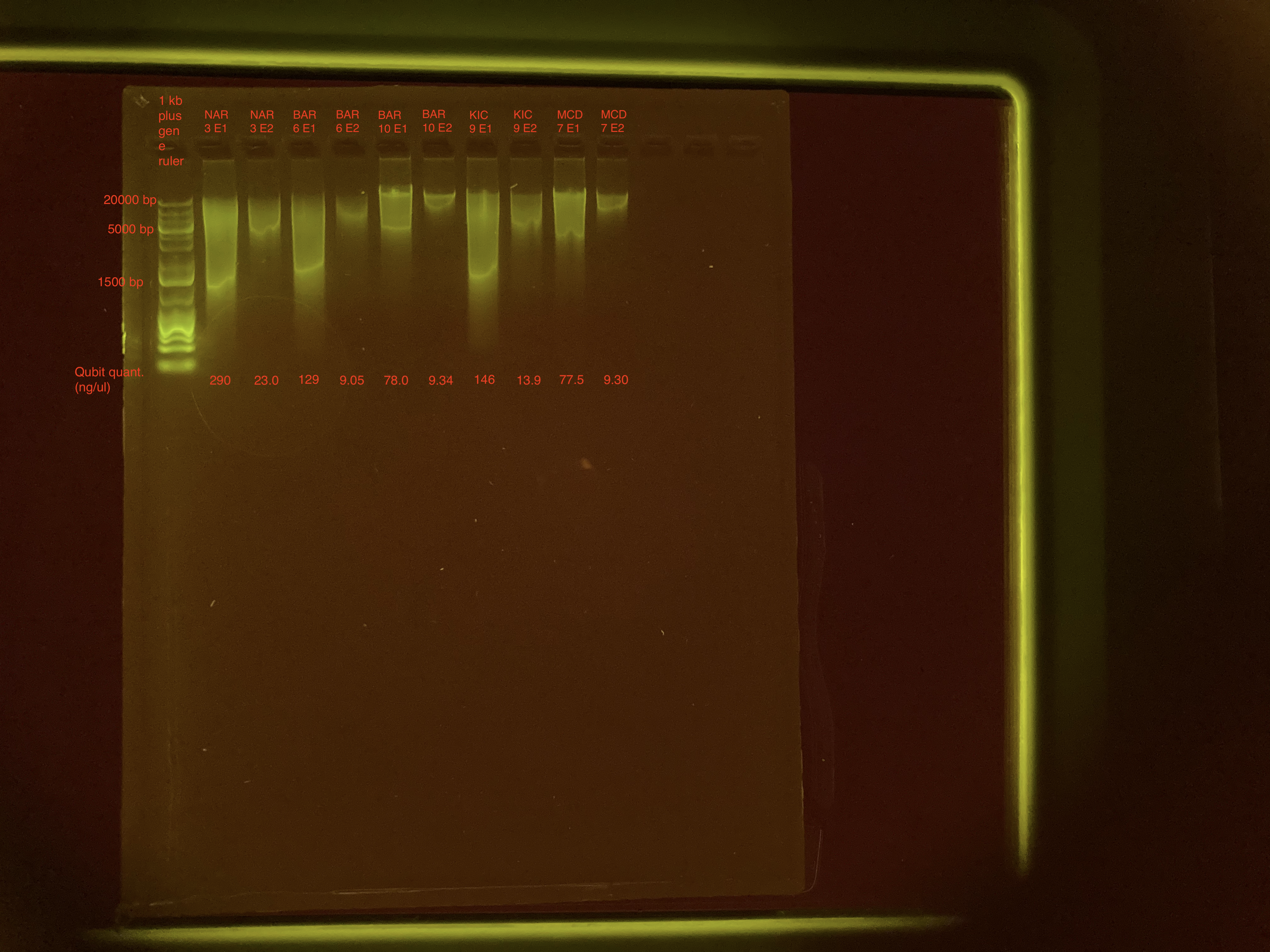
Same as before, first elution has smaller fragments than second elution. Gel doesn’t look super pretty. I may run two or three of these samples on the TapeStation just to make sure everything is good. I also think I’ll run the gel longer next time.
