Narragansett Bay Adult Oyster DNA Re-Extraction - Part 4
DNA Extraction of Adult Oyster Tissue - Re-extractions Part 4
Re-doing DNA extractions for samples where there either wasn’t enough DNA from the first extraction or it was low quality DNA.
DNA Extractions
Completed on April 18, 2021
Zymo Research Quick-DNA Miniprep Plus used for DNA extractions of adult oyster tissue
- Pull samples out of -80 freezer and put on ice
- NAR_8 M2
- GHP_2 M2
- BAR_8 M2
- MCD_10 G2
- Pull Proteinase k out of upright -20 freezer in Puritz Zymo Reagents box and put on ice
- Pull out Type II DI water, 10X PBS solution, Blue solid tissue buffer, and Nuclease-free water
- In original sample tube, add 90 ul of Type II DI water and 10 ul of 10X PBS (Phosphate Buffered Saline) solution to create 1X PBS solution soak
- Vortex and spin down in mini centrifuge
- Let soak for 5-10 minutes while preparing next set of tubes
- In 2 ml Homogenization Tubes with ceramic beads, add 190 ul of Blue Solid Tissue Buffer and 190 ul of Nuclease-free water
- Vortex and spin down
- Sterilize forceps with 10% Bleach, Type II DI Water, and 70% EtOH
- Use forceps to transfer tissue piece from original tube with PBS solution to 2 ml Homogenization tube with ceramic beads at solid tissue buffer and nuclease-free water
- Sterilize forceps before each sample
- Setup tissuelyser II
- Make sure to balance both tube racks
- Homogenize tissue samples in tissuelyser II for 2 minutes at 30 Hz
- This will create a ton of bubbles in the sample tubes
- Let the samples sit for about 3 minutes then spin down on tabletop centrifuge at 13000 rcf for 1 min to bring down bubbles
- I also spun down on minifuge
- Add 20 ul of Proteinase k to each sample
- Vortex for 10 seconds then spin down on mini Centrifuge
- Put samples in thermomixer at 55 deg C at 1200 rpm for 30 minutes
- Halfway thru, spin down samples on tabletop centrifuge at 13000 rcf for 1 min
- I also reduced speed to 1000 rpm halfway thru
- Halfway thru, spin down samples on tabletop centrifuge at 13000 rcf for 1 min
- Make new set of labeled 1.5 ml tubes
- After 30 minute incubation, place all samples in tabletop centrifuge and spin at 13000 rcf for 2 minutes
- Transfer all supernatant (400 ul) to the newly labeled 1.5 ml tubes
- Make 1.5 ml tube of 10 mM Tris HCl pH 8 and place in thermomixer at 70 deg C
- Set up tubes for extraction - 1 yellow spin column inside a collection tube for each sample - label lid of spin column
- Get liquid waste beaker from sink near -80 freezers
- Add 2 parts volume (800 ul) of Genomic Binding buffer to each tube
- Vortex for 5 seconds and spin down in mini centrifuge
- Add 800 ul of sample to their labeled yellow spin column
- Centrifuge spin columns at 13000 rcf for 1 minute
- Pour off flow through in liquid waste beaker
- Put spin columns in same collection tubes
- Add remaining liquid (400 ul) from each sample to labeled yellow spin column
- Centrifuge spin columns at 13000 rcf for 1 minute
- Pour off flow through in liquid waste beaker
- Transfer spin columns to new collection tubes and discard of old collection tubes
- Add 400 ul of DNA pre-wash buffer to each spin column
- Centrifuge at 13000 rcf from 1 minute
- Pour off flow through in liquid waste beaker
- Place spin columns in same collection tubes
- Add 700 ul of g-DNA wash buffer to each spin column
- Centrifuge spin columns at 13000 rcf for 1 minute
- Pour off flow through in liquid waste beaker
- Put spin columns in same collection tubes
- Add 200 ul of g-DNA wash buffer to each spin column
- Centrifuge spin columns at 13000 rcf for 1 minute
- Make final 1.5 ml tubes - label lid with sample id, elution #, and DNA; label side with initials, date of extraction, sample id, elution #, DNA, and C. virginica
- Make 2 tubes for each sample - splitting the two elutions into two separate tubes
- Transfer spin columns to first set of labeled 1.5 ml tubes
- Pour off flow through in liquid waste beaker and discard collection tubes
- Take warmed 10 mM Tris HCl pH 8 out of thermomixer
- Add 30 ul of warmed 10 mM Tris HCl pH 8 to each spin column by dripping directly over the filter without touching it
- Immediately place tubes in centrifuge with all the lids of the 1.5 ml tubes facing the same direction and centrifuge at MAX speed for 1 minute
- No incubation period for the first elution
- Take tubes out - DO NOT pour off liquid, keep in tube
- Transfer spin columns to second set of labeled 1.5-ml tubes, add 100 ul of warmed 10 mM Tris HCl pH 8 to each spin column by dripping directly over filter without touching it
- Put first set of 1.5-ml tubes with the first elution on ice
- Incubate for 15 minutes
- Centrifuge at max speed for 1 minute
- Take out spin columns at discard
- Make labeled PCR strip tubes - 1 for each elution = 2 per sample
- Add 1 ul of DNA and 4 ul of nuclease-free water to the respective PCR tube for agarose gel
- 1 ul will be taken directly from 1.5-ml tube for Qubit
28 ul remaining in all E1 1.5 ml tubes; 98 ul remaining in all E2 1.5 ml tubes
Qubit dsDNA BR assay
The captured pools were quantified following Qubit protocol for BR DNA
| Sample | Avg ng/μl |
|---|---|
| Std 1 | 154 RFU |
| Std 2 | 18739 RFU |
| NAR_8 E1 | 219 |
| NAR_8 E2 | 14.6 |
| GHP_2 E1 | 145 |
| GHP_2 E2 | 9.12 |
| BAR_8 E1 | 175 |
| BAR_8 E2 | 15.8 |
| MCD_10 G E1 | 59.4 |
| MCD_10 G E2 | 2.39 |
Agarose Gel Electrophoresis
The DNA quality and size were assessed following Agarose Gel Protocol for a small 1% gel.
- Only 1 ul of DNA for each sample were used
- 4 ul of nuclease-free water added to bring volume up to 5 ul
- Gel was run for 1 hour
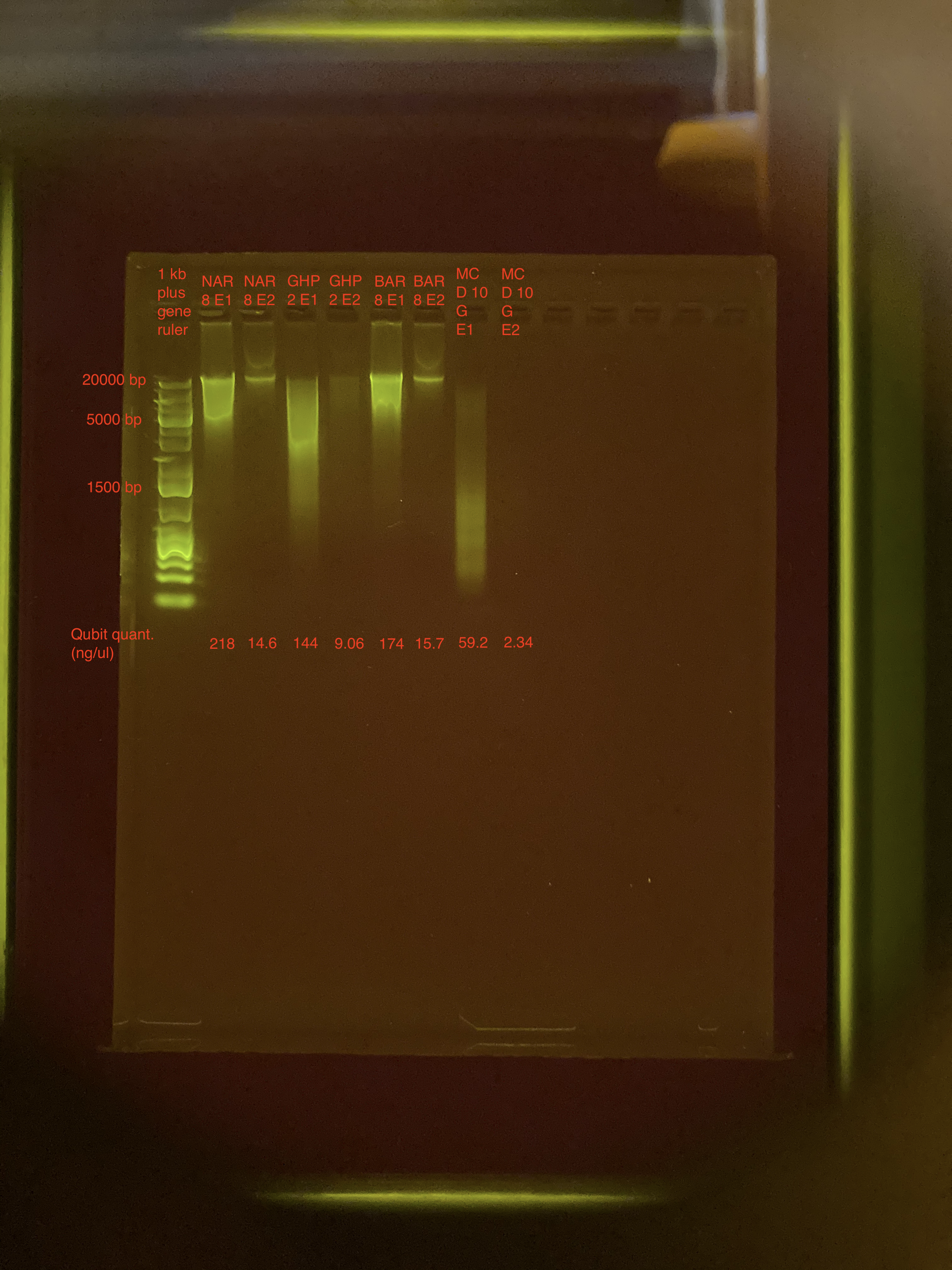
Overall, gel looks good.
Written on April 18, 2021
